Hello,
The following are the SOP's for post-op thermal and nutritional support that UCSD will use.
We are open to comments.
Thank you!
Standard Operating Procedure (SOP) for mouse MCAO Post-stroke thermal support
Difference with humans, all post-stroke mice lose body temperature to as low as 30-32C during the first 24-48 hours (probably because of small body mass). This SOP is based on a previous study showing that the post-stroke “spontaneous hypothermia” offers significant brain protection in the mouse monofilament model (Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004 Jul;35(7):1720-5).
When testing a cerebroprotective intervention, controlling mouse body temperature at the same level between treatment groups may confirm that the cerebroprotection is not due to the post-stroke hypothermia.
- Place a thermal support pad setting at 28 C below 1/3 of the home cage bottom area for the first 48 hours.
- Monitor/record temperature at 2, 4, 24, and 48 h, this can be done with a Non-Contact Infrared Thermometer with Laser Sight (i.e. Catalog# CODA-IRT from Kent Scientific)
- Make adjustments to the thermal support pad setting temperature or coverage area under the home cage in a pilot study
- The temperature management should be identical among all mice in the study.
Standard Operating Procedure (SOP) for mouse MCAO Post-stroke nutrition support
Differing from humans, rodents after stroke lose body weight significantly. This SOP is based on a previous study showing that post-stroke nutrition support can significantly increase the survival rate from 40.9% to 85% at 14 days post-MCAO using the mouse monofilament model (Lourbopoulos et al. Inadequate food and water intake determine mortality following stroke in mice. J Cereb Blood Flow Metab. 2017 Jun;37(6):2084-2097).
- Post-op mice will be given a combination of (i) oral feeding of a diluted jelly diet via with a syringe; (ii) jelly diet ad libitum in a petri dish; and (iii) soft pellets ad libitum in a petri dish.
- The oral feeding of the diluted jelly diet will be done 3 times per day until the mice recover 85% of the basal body weight. In brief, the diluted jelly diet will be made using one portion of the jelly diet (Bio Serv F0550SP) and three portions of water. The lab staff takes 1 ml of the diluted jelly food with a syringe, restrain awake mice by hand, and delivers the diluted jelly food to the mouse oral cavity in a stepwise manner, trying to deliver one drop (about 50 ul) at a time, until 1 ml diluted jelly food is given.
- The soft pellets (BIO SERV # F3580-1) will be made by soaking the food pellets in water and be provided in a 5 cm diameter petri dish on the mouse cage floor.
- The jelly diet (BIO SERV # S5769-TRAY) will be provided in a 5 cm diameter petri dish on the mouse cage floor.
- The combination of post-op nutrition support will be provided until the mice recover 85% of the basal body weight. The lab staff will check the Petri dishes with soft pellets and jelly diet every 12 hours and refill them when needed.


Heating pad
You may use Gaymar heating pump and multiple heating pads (in series) setting at temperature at 27-28C. The temperature setting and heating pad coverage under the home cage need to be tested in a pilot study in each lab, as the ambient temperature and ventilation may be different among labs.
post-stroke time points for measuring body temperature
We may discuss if we can reduce the measurement times to one or two time points (4 and 24 h) post-stroke; mouse post-stroke hypothermia occurs between 1-24 h post-stroke
IACUC satellite housing for management of mouse post-stroke hypo
Management of mouse post-stroke hypothermia is one of justifications for our approved IACUC satellite housing protocol at UCSD
Updated protocols for discussion
Standard Operating Procedure (SOP) for mouse MCAO Post-stroke thermal support
Difference with humans, all post-stroke mice lose body temperature to as low as 30-32C during the first 24-48 hours (probably because of small body mass). This SOP is based on a previous study showing that the post-stroke “spontaneous hypothermia” offers significant brain protection in the mouse monofilament model (Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004 Jul;35(7):1720-5).
When testing a cerebroprotective intervention, controlling mouse body temperature at the same level between treatment groups may confirm that the cerebroprotection is not due to the post-stroke hypothermia.
Note: infrared thermometers have a larger margin of error than rectal probes, albeit only slightly. The margin of error for measurements with rectal probes is ± 0.1 °C, whereas for infrared thermometers, it is ± 0.2 °C.
Hold the trigger to measure the temperature. NOTE: Mice tend to move; take care in measuring the same location of the body consistently by taking temperature measurements when mice are relatively less mobile.
Standard Operating Procedure (SOP) for mouse MCAO Post-stroke nutrition support
Differing from humans, rodents after stroke lose body weight significantly. This SOP is based on a previous study showing that post-stroke nutrition support can significantly increase the survival rate from 40.9% to 85% at 14 days post-MCAO using the mouse monofilament model (Lourbopoulos et al. Inadequate food and water intake determine mortality following stroke in mice. J Cereb Blood Flow Metab. 2017 Jun;37(6):2084-2097).
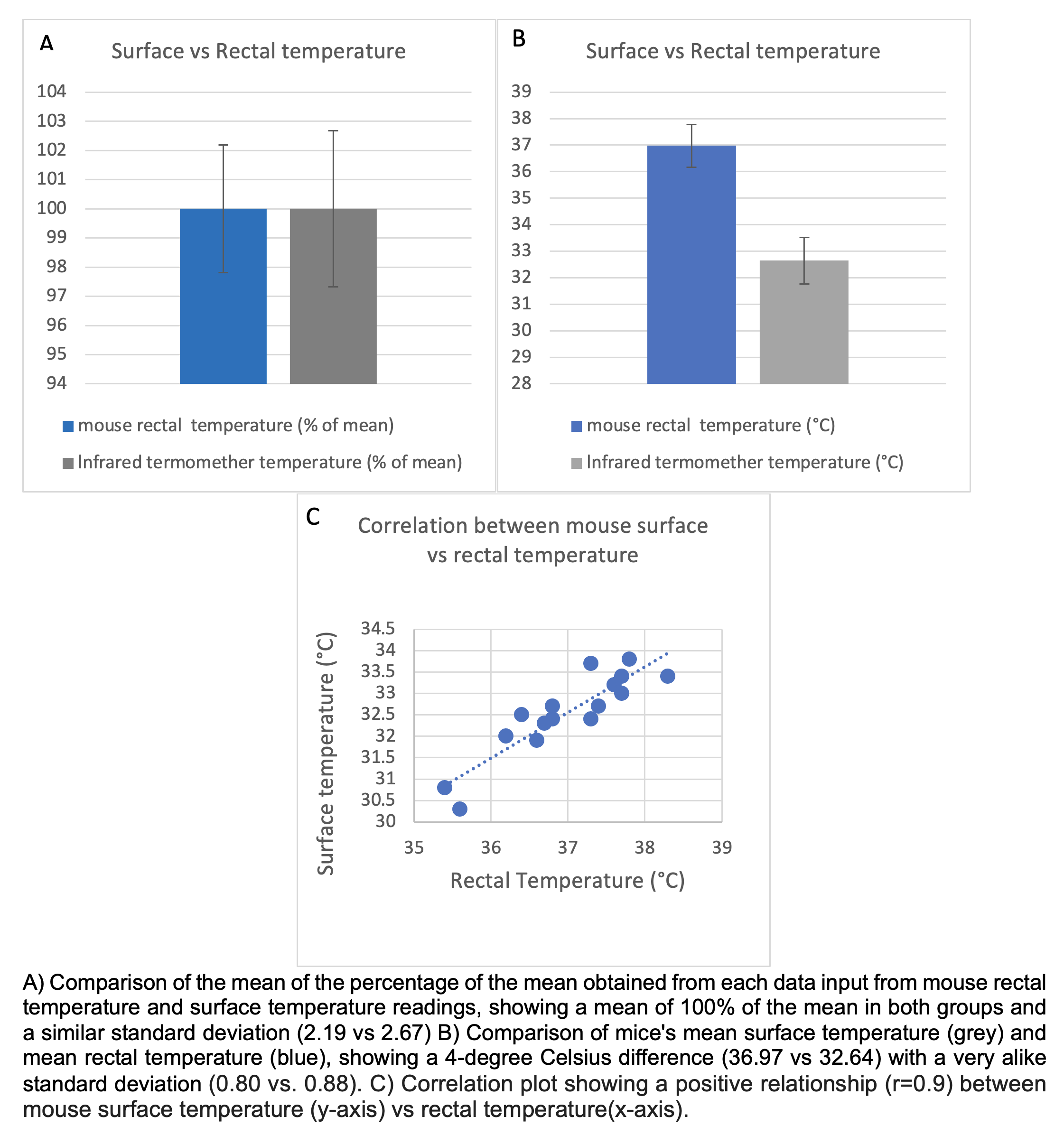
Mouse rectal Temperature vs Surface Temperature
Objective: The objective of this test was to understand the relationship between body surface temperature measured with an infrared thermometer and rectal temperature measured with a mouse rectal temperature probe in a sample of 17 mice.
Method: Rectal and body surface temperatures were measured in 17 mice aged between 2 to 3 months. Rectal temperature was obtained using a probe connected to a thermocouple thermometer, which was inserted 2 cm into the rectum with the aid of water-based lubricant jelly. The probe was left in place for approximately 1 minute until the temperature stabilized without any fluctuations. Following the rectal temperature measurement, body surface temperature was measured using an infrared thermometer. The mice were placed on a horizontal bar grid surface, allowing their frontal limbs to grasp the bars. The operator gently lifted the mouse tail to aim and shoot the thermometer at the abdominal area between the second nipples from about 1.5 cm away from the skin.
Data Analysis: Mean and standard deviation (SD) were calculated for each temperature measurement group. Additionally, the percentage of the mean was calculated for each group, and the difference between rectal and surface temperatures was determined. Pearson's correlation coefficient was used to assess the relationship between the two sets of temperature data.
Results: The mean rectal temperature was 36.97 +/- 0.80°C. The mean surface temperature was 32.64 +/- 0.88°C. The correlation coefficient between the rectal and surface temperature measurements was 0.9 (p = 0.001). The mean difference between the two groups was -4.33+/- 0.40°C.
Conclusion: Evaluating mouse temperature is crucial for assessing post-MCAO recovery, particularly during the first 24 hours post-MCAO. This study confirms the validity of using an infrared thermometer to measure surface temperature in mice. The standard deviations obtained using the infrared thermometer were comparable to those obtained with the traditional rectal probe (0.80 vs. 0.88). Although a temperature difference of approximately 4 degrees (-4.33°C) was observed, the strong correlation between the body surface and rectal temperature measurements suggests that the infrared thermometer can effectively and accurately detect mouse body temperature changes in the room temperature ambient environment. This non-invasive method reduces the reading time (from 60 seconds to 15 seconds) and minimizes animal stress, making it a valuable tool for obtaining temperature data.
We suggest documenting the animal abdominal surface temperature data at the same time points as those for collecting body weight data in post-MCAO animals.