As you all know, the mouse filament MCAO is not a pure MCAO. Sometimes, this MCAO procedure results in MCAO + PCAO (posterior cerebral artery occlusion). These mice will only survive for a few days. That is why the mortality is high in mouse MCAO and an 802212 filament is recommended. The key in this model is not to block the PCA. Currently, the ischemic duration is limited to 60 minutes. The SPAN1 results indicated the average lesions at 30 days post-stroke were not large because severely injured mice died. If we use the same SOP in this pilot study, what we will expect?
Do we need an alternative mouse long-term survival MCAO model? We have a transcranial MCAO model, in which ischemic duration can be up to 6 hours. The mortality is very low and the lesion is moderate. It requires surgical skills. If you are interested, we can share the surgical procedure with you.
The rat MCAO does not have such a problem. The ischemic duration can be increased to 90 minutes. At least we can use this rat model first. Behavioral changes should be different between 20 minutes and 90 minutes.
Thank you Huaxin. There is more to the mouse MCAo. We did use LDF monitoring. We left the CCA open, which lessens the severity. The mortality in young mice was quite low. We do have alot of data that blocking the pCom changes the model alot, and we are going to change to the shorter filament. Also, we wake up the animal between occlusion and de-occlusion. this lessens the severity of the injury due to lower anesthesia time.
We believe that 20 minutes of MCAO is very mild compared to 60 minutes of MCAO in mice. However, the differences were not found in behavioral tests and MRI images in SPAN1, right? Two possibilities: 1) the ischemic lesions in both groups were similar at the end of the experiment (probably more mice died when they had 60 minutes of MCAO), and 2) the behavioral tests used in SPAN1 were not good for long-term survival study. You believe it is #2 so the pilot study is for testing new behavioral tests. What about if it is #1?
Can we just do rat MCAO first and test the behavioral tests used in SPAN1 to further verify these behavioral tests? Cylinder test, tape removal, and foot fault are supposed good behavioral tests for long-term stroke studies.
For embolic mouse MCAO, I found that Iowa's group only let the mice survive for 7 days and Augusta's group let the mice survive for 48 hours. Probably, we should use the rat model as well.
I agree with you and Anil that we should do both rats and mice in the pilot to ensure we choose behavioral tests that are sensitive and reproducible across labs in both species. One finding that Cenk reported from his analysis was that waking mice up during occlusion actually led to smaller strokes (not larger as we expected from neuroprotection from anesthesia)- we think that is because the filament may have moved a little it as animals were moving around, but really don't know for sure.
We do have foot fault data from our grid walking setup from SPAN 1.0 that we should look at closely.
I'm very interested in the extended neurological scoring you developed, Y maze, and the phenotype box that Bingren showed.
After seeing the control cohort data and other ongoing projects in the lab where we used the SPAN protocol, I am strongly opposed to awake state during MCAO. The cause of smaller infarcts when you wake them up is likely premature reperfusion due to filament movement and leak as well as higher arterial pressures in animals awakening with sutured incisions etc. despite pain control.
Temperature is another factor affecting the stroke outcome. If the temperature is not controlled during awake MCAO, we have to make sure the heating will be switched off and on at the same pace in all mice.
We had one experiment in which we controlled the temperature during awake MCAO. A soft probe was implanted for temperature control and animals carried a swivel device so the temperature probe would not be pulled out. The infarcts were larger when the rats were kept awake during MCAO. We had to decrease the ischemic duration from 90 minutes to 75 minutes in that experiment.
We invariably saw smaller infarcts in awake animals. Very recently, for a different project, we used the SPAN filament MCAO protocol but kept the animals awake and MCAO for 90 minutes. Infarcts were smaller than when we kept them anesthetized and only 60 min MCAO.
Possible reasons are filament instability causing premature reperfusion during animal movement and hypothermia (more relevant for mice) while awake because we cannot control the temperature, and they are not as physically active as before the stroke.
In rats, the situation may be different.
PERHAPS SOMETHING WE COULD TEST IN THE PILOT? Half of the rats and mice at each site could be awake and half anesthetized during MCAO.
We know that blood pressure is higher in the awake state. If the filament you used only induces a small infarct, another filament coated with a little long silicone should be considered in awake mice. We can test it in the pilot as well.
I agree on all the issues with small infarcts when awakening the mice. I think the main drawback to keeping animals asleep the whole time is the number of surgeries per day that can be done in the rats given the 2 hour occlusion time. Agree with Huaxin, about the potential filament size confounder. We should look at that SPAN 1.0 data carefully together as we make these decisions. We probably have the data to answer the question without including as another variable in the pilot
Here is some feedback and queries from MGH regarding the 48-point scoring system upon viewing the video:
1. Many of the scoring points are subjective, not easy to evaluate, difficult to differentiate between different grades (e.g., somnolent vs calm).
2. The test will be done in mice but videos are from rats. Will it be as easy to evaluate mice for the same scoring points?
3. The test is labor-intensive, takes too long.
4. Do we need baseline assessment or will we assume perfect performance at baseline? Do we need to wait a certain time between different sections of the test in the same animal?
We will be performing a mock exam in one mouse as requested and we will let you know how long it takes in our hands and if we have any additional feedback.
I vote to do the rat model in the pilot study. Rats do not have the PcomA issue so the coated silicone can be a little longer so we can do the awake MCAO if we want. In the mouse model, we use the filament coated only with short silicone (do not like block the blood flow to PCA) so the blood flow to MCAO will not be well blocked if the filament is shifted during ischemia in the awake state. More importantly, the mortality is low in the rat model.
After watching the behavioral test video. Here are some comments from Augusta sites:
1. Scoring systems were exhaustive and time-consuming. Their activity may change early morning vs late noon. Also, the behavioral activity grades here are not mentioned whether it is performed in regular or reverse light cycles of rodents. So, counting all these 48-point grades look time-consuming and not easy to evaluate. If we plan to do more behavioral testing mice will get exhausted and stressed. In most of the tests, we need to handle mice or rats which is not good if we need many tests in a day. If we want to use this 48 points scoring we need to minimize the number of testing. From the suggested testing we can do fewer tests such as:
General status: Spontaneous activity and body symmetry
The spontaneous activity may change early morning or late afternoon. However, when you put them in a new clean cage, they always walk around. For beginners, it may take time to get it done. In fact, just a few steps: watch or test them inside a new cage, watch or test them on the table, beam walking, and screen climb. These activities are essential and not affected by the light cycle.
Have you done any long-term survival studies in mice using 90 min filament MCAO model? If yes, please share some information about the filament size, body temperature, and mortality.
The mortality rate could be high in long-term survival studies in mice if using 90 min filament MCAO model. However, we have not done this yet in mice.
I have done a study comparing 30, 60, 90, 120 and 3h, 6h and 12h occlusion times in a rat MCAO model. We use heat blunted suture and we occlude the CCA but we wake them up during occlusion. we had one death during stroke, one in the 30min group, and one in the 3h group, but no deaths in the other groups. Rats are very hardy.
All of us have no problem with 90 min MCAO in rats. However, it is not easy to do it in mice. We use a transcranial MCAO mouse model for long-term survival experiments. The model can be used for several hours of ischemia and the mortality is almost zero. I do not know if you like to use it here.
Here is the feedback from MGH regarding the 48-point test.
Sanem and Taka did one mouse (C57 day 2 after 1h filament MCAO). The test took 60min to run it, also 20-30 min habituation plus recording every video may take a total of 90 min.
Concerns & Thoughts:
Do we need to record every video separately for 14 tests. If we need, it will consume more time.
1 person may not be enough to do it, 2 people may be needed. 2 stations with cameras may be needed.
In total test is too long, animal gets exhausted and needs a break after each test. This may prolong the total time.
Beam walk test is performed 3 times but what happens to scoring if the animal does not fall in first but falls on second or third attempt.
We think sensory tests are not really evaluating sensory because it is not easy differentiate if the animal is slowly moving because of loss of sensation or decreased motor performance.
Sensory test is not easy to assess because mouse has small limbs that you can’t easily do the test and evaluate like rats.
We did not have fibrinogen on time so we did without.
Blood sample was collected from both a vein and an artery. Venous blood clot was stable, arterial tended to fragment easy. Yellow tape is under the arterial clot. Washed 15 times. It seems easy to make the clot but whether we can successfully suck into the catheter and inject into ICA remains to be seen.
Ligia and I just completed testing of the Duke behavioral battery on a healthy WT young male mouse. Our observations and recommendations are listed below. Overall, the test seems really promising and a much more accurate reflection of clinical outcomes, but the challenges of translating everything from rats to mice, as well as the time/effort that would be required both to perform and to rate the videos, is pretty high.
- Only tried on a non-stroked animal, so many of the tests are much more difficult to do than they would be with an animal who has stroke-related deficits. We should probably try it again with a stroked animal and compare the two.
- Testing clearly requires two camera angles — one from the top, and one from the side. Each test is better viewed from one or the other. Would maybe make sense to film all the 'from the top' tests at once and then all the 'from the side' tests at once, if moving around the order of the tests is allowed?
- Took us 25 minutes from start to finish for one animal, while consulting video for how to do tests and not doing the complex motor tests. Would guess that 15 min per animal is reasonable once someone is trained, especially if they don't have to score as they go.
- A lot of ambiguity on how long each test should take, how many trials are needed, etc. A detailed SOP would be incredibly useful.
- Mouse was clearly very stressed about midway through the tests, which is a concern. The video recommends waiting a few minutes for the mouse to calm down, but this would need to be balanced against increasing the length of evaluation.
- Many of the tests are done on an elevated countertop. Would need to design a 3-sided box or some other structure so that the mice don't move out of camera range.
OBSERVATIONS ON SPECIFIC TESTS ARE BELOW
General Status:
- Seems super easy to do General Activity and Body Symmetry tests — can film from top. They suggest recording for 5 minutes, but we both feel you could get the same info with a 1-2 min video.
- Gait Walk test needs lateral recording and some sort of box so animals don't escape.
Motor Tests:
- Front Limb Symmetry and Front Limb Circling could possibly be combined into one recording. More difficult to do with mice because holding them at the exact right height is challenging. Needs lateral recording.
- Circling test relatively straightforward, would be recorded from the top.
- Hind Limb Placement test seems really difficult to do with a mouse - feels like they're going to fall when you put them on, and is hard to assess. Also would require a complicated camera setup.
Complex Motor Tests:
- Tried a modified version of the Screen Climb test using one of the grid walk grids. Seemed to work pretty well. Couldn't try out the Wooden Beam test.
- Would need standardized, non-wood equipment for both the Screen Climb test and Wooden Beam test if we're going to use it in Gold Standard.
Sensory Tests:
- Very, very difficult to assess with a non-stroked animal. The mouse constantly runs away, making evaluation or repeated trials quite challenging. Difficult to isolate the effect of sensory stimulation from general skittishness.
- A lot harder to separate facial from whisker stimulation in mice than in rats, especially if the animal is healthy.
- We used a Q-tip, rather than the blunt 30G needle they recommend. Seemed to elicit a response, but the relative sharpness of the needle may be a more salient stimulus.
- Forelimb and hindlimb tests are probably best to film laterally, while the face, vibrissae, and back tests can all be filmed from the top.
Ligia and I just completed testing of the Duke behavioral battery on a healthy WT young male mouse. Our observations and recommendations are listed below, and you can find videos of some of the testshere. Overall, the test seems really promising and a much more accurate reflection of clinical outcomes, but the challenges of translating everything from rats to mice, as well as the time/effort that would be required both to perform and to rate the videos, is pretty high.
- Only tried on a non-stroked animal, so many of the tests are much more difficult to do than they would be with an animal who has stroke-related deficits. We should probably try it again with a stroked animal and compare the two.
- Testing clearly requires two camera angles — one from the top, and one from the side. Each test is better viewed from one or the other. Would maybe make sense to film all the 'from the top' tests at once and then all the 'from the side' tests at once, if moving around the order of the tests is allowed?
- Took us 25 minutes from start to finish for one animal, while consulting video for how to do tests and not doing the complex motor tests. Would guess that 15 min per animal is reasonable once someone is trained, especially if they don't have to score as they go.
- A lot of ambiguity on how long each test should take, how many trials are needed, etc. A detailed SOP would be incredibly useful.
- Mouse was clearly very stressed about midway through the tests, which is a concern. The video recommends waiting a few minutes for the mouse to calm down, but this would need to be balanced against increasing the length of evaluation.
- Many of the tests are done on an elevated countertop. Would need to design a 3-sided box or some other structure so that the mice don't move out of camera range.
OBSERVATIONS ON SPECIFIC TESTS ARE BELOW
General Status:
- Seems super easy to do General Activity and Body Symmetry tests — can film from top. They suggest recording for 5 minutes, but we both feel you could get the same info with a 1-2 min video.
- Gait Walk test needs lateral recording and some sort of box so animals don't escape.
Motor Tests:
- Front Limb Symmetry and Front Limb Circling could possibly be combined into one recording. More difficult to do with mice because holding them at the exact right height is challenging. Needs lateral recording.
- Circling test relatively straightforward, would be recorded from the top.
- Hind Limb Placement test seems really difficult to do with a mouse - feels like they're going to fall when you put them on, and is hard to assess. Also would require a complicated camera setup.
Complex Motor Tests:
- Tried a modified version of the Screen Climb test using one of the grid walk grids. Seemed to work pretty well. Couldn't try out the Wooden Beam test.
- Would need standardized, non-wood equipment for both the Screen Climb test and Wooden Beam test if we're going to use it in Gold Standard.
Sensory Tests:
- Very, very difficult to assess with a non-stroked animal. The mouse constantly runs away, making evaluation or repeated trials quite challenging. Difficult to isolate the effect of sensory stimulation from general skittishness.
- A lot harder to separate facial from whisker stimulation in mice than in rats, especially if the animal is healthy.
- We used a Q-tip, rather than the blunt 30G needle they recommend. Seemed to elicit a response, but the relative sharpness of the needle may be a more salient stimulus.
- Forelimb and hindlimb tests are probably best to film laterally, while the face, vibrissae, and back tests can all be filmed from the top.
The animal we studied (as above) showed mild deficits (many sub-score 1-2). TTC showed good stroke volume. If stroke sizes are smaller, it maybe difficult to judge the score.
Yes. Most stroke animals have a sub-score of 1-2. In our 90 min MCAO rat experiment, the mean neurological scores at 7 days post-stroke were 16 in the vehicle group, and 10 in the treatment group. At 28 days post-stroke, the scores were 12 and 7 in the vehicle and treatment groups, respectively.
I shared link to a google drive folder with videos from our piloting of the Duke protocol with Jessica and Karisma, hopefully they can share with the emails the PIs are able to access google drive with (I think some were using different emails due to university restrictions and I don't know who or what they are).
Our lab can do the mouse MCAO model using both awake or anesthetized mice. However, the mouse MCAO model is more reproducible in anesthetized mice than in awake mice. If a drug testing in an individual lab, I would highly recommend using anesthetized mice and monitoring/measuring the MCA blood flow during the entire peri-MCAO periods. However, the multiple site testing might overcome the reproducibility issue as more mice will be used for the study.
Thank you for hosting the stimulating discussion of the span SOPs. Below is our consideration of the filament occluder size: The monofilament suture occluder size is important in producing a reproducible MCAO and reperfusion. Minor alterations in the suture silicone coating diameter can greatly affect stroke brain injury volume and reproducibility in this model. In our studies, we use a body weight-to-diameter scale method; the lower the body weight in animal, the smaller of a silicone coating tip diameter is used. For the mouse model, our mouse surgeons prefer the Syneture™ Monosof™ SN5696G 6-0 suture because of the suture quality and consistency. The suture tip is coated with silicone to different sizes in diameter. For example, a 0.22-0.23 mm diameter range is used for 3 months old B6 male mice (23-28 g). A 0.19-0.21 mm diameter range is used mostly for 3 months old B6 female mice (17-22 g). When a larger size of suture tip is used for a smaller animal, the suture tip may not reach to the MCA base or punch through the vessel wall before reaching the MCA origin, resulting in either incomplete occlusion of MCA or subarachnoid hemorrhages.
In our experience, smaller mouse body weight variations in a range of 23-28g, the 0.21-0.23 mm tip width may not make any difference in the infarct volume. However, when mice are significantly smaller such as female mice, a small tip width may offer more reproducible result.
It is very useful to use a needle laser doppler probe to monitor the MCA blood flow while inserting the monofilament occluder to the MCA base as the MCA blood flow sudden drop can clearly indicate that the occluder reaches the MCA base. When a filament occluder tip is >0.22 mm for smaller mice such as female mice (body weight about 19-20 g), the occluder may not be able to reach to the MCA base. This can be seen by monitoring the MCA blood flow as the MCA blood flow will not drop even when the inserting resistance is felt by the surgeon. In this situation, inserting with more force will likely punch through the vessel wall before reaching the MCA origin, resulting in either incomplete occlusion of MCA or subarachnoid hemorrhages.
Two mice had 20 min MCAO and only one had a small infarct. Another two mice had 30 min MCAO and both has a small infarct. It seems like we should use 30 min MCAO in the pilot study.
Nicely done, Huaxin. May I ask if you anesthetized the mice and continuously monitored the MCA blood flow during the peri-MCAO periods (pre-, during, and post-MCAO)?
Yes. Mice were anesthetized with 1.5% isoflurane in 30% O2 and 70% N2O. We used the 602223 filaments and monitored blood flow during ischemia. The blood flow was reduced to 40% of the baseline when CCA was open.
40% of MCA blood flow during MCAO related to the pre-ischemic baseline? Is the 40% too high? We temporally clamp/close CCA during MCAO and the MCA blood flow during MCAO is below 10% of the pre-ischemic baseline.
Videos recorded far away from the subject & are not easy to score. Mouse 1 circling video is too short, and the mouse disappears from the video.
General status videos:
Difficult to differentiate between scores, 1/2 points + 3/4 points, which looked very similar to us.
We suggest a new scoring scale (new grades in blue.) 5 minutes is too long, 1–3-minute duration should be enough.
0 points: Normal (0)
1 Point: Calm (1)
2 Points: Somnolent (1)
3 Points: Stuporous (2)
4 Points: No spontaneous movement (2)
Symmetry videos:
Body symmetry videos are similar to circling/gait videos. Only forelimb symmetry can be checked.
Grading (Gait) videos: Videos recorded at too great of a distance. Not easy to score and observe the animal.
Circling bench top videos: Mouse 1 video was short, could not even evaluate the circling. During the gait video, circling can be evaluated with no need to separate the tests.
Circling hold tail: This is similar to the forelimb symmetry test. If the mouse is circling asymmetry will occur.
Hind limb placement: Mouse is much lighter than a rat, will be difficult to conduct this testing.
Screen climbing: MGH team approves of this testing strategy. We’ll need specifications of the testing apparatus (gap size, angle, height) to prepare MGH spaces for this test. The 0-4 score looks good.
Beam walk: We will require beam specifications. Beam walk test must be performed 3 times – how will we conduct scoring if the animal does not fall during first trial? What if animal falls on second or third attempt?
Forelimb touch and Hindlimb touch: This sensory test is not easy to assess because, unlike rats, mice have small limbs. This complicates graders’ ability to score videos. Videos are recorded from too great a distance and are not easy to evaluate. Sensory testing tools must be standardized- MGH staff noted differences between the equipment used by Yale and Duke.
Trunk, vibrissae, and face touch: Again, videos are taken from too great a distance. Not easy to evaluate. We think this test is helpful to use. Video recording should be standardized to maximize evaluation success.
We propose the Garcia 18-point scoring test, published in 1995, as an alternative to the 48-point testing proposed by SPAN CC. MGH believes that the Garcia 18-point test will cover all aspects of the 48-point scoring test.
The circling behavior is included in the 48-point score system. We took one old experiment, mixed all treatment animals, and analyzed the circling behavior separately. The circling behavior was associated with the infarct size (p=0.03). However, the neurological score represented the infarct size better.
There was a significant infarct volume difference between these two groups. However, the corner test result was not significant. Is there a clear answer to this? The corner test will serve as the primary outcome of our pilot study, as we discussed in Friday's meeting.
Mouse MCAO
As you all know, the mouse filament MCAO is not a pure MCAO. Sometimes, this MCAO procedure results in MCAO + PCAO (posterior cerebral artery occlusion). These mice will only survive for a few days. That is why the mortality is high in mouse MCAO and an 802212 filament is recommended. The key in this model is not to block the PCA. Currently, the ischemic duration is limited to 60 minutes. The SPAN1 results indicated the average lesions at 30 days post-stroke were not large because severely injured mice died. If we use the same SOP in this pilot study, what we will expect?
Do we need an alternative mouse long-term survival MCAO model? We have a transcranial MCAO model, in which ischemic duration can be up to 6 hours. The mortality is very low and the lesion is moderate. It requires surgical skills. If you are interested, we can share the surgical procedure with you.
The rat MCAO does not have such a problem. The ischemic duration can be increased to 90 minutes. At least we can use this rat model first. Behavioral changes should be different between 20 minutes and 90 minutes.
Thank you Huaxin. There is…
Thank you Huaxin. There is more to the mouse MCAo. We did use LDF monitoring. We left the CCA open, which lessens the severity. The mortality in young mice was quite low. We do have alot of data that blocking the pCom changes the model alot, and we are going to change to the shorter filament. Also, we wake up the animal between occlusion and de-occlusion. this lessens the severity of the injury due to lower anesthesia time.
We believe that 20 minutes…
We believe that 20 minutes of MCAO is very mild compared to 60 minutes of MCAO in mice. However, the differences were not found in behavioral tests and MRI images in SPAN1, right? Two possibilities: 1) the ischemic lesions in both groups were similar at the end of the experiment (probably more mice died when they had 60 minutes of MCAO), and 2) the behavioral tests used in SPAN1 were not good for long-term survival study. You believe it is #2 so the pilot study is for testing new behavioral tests. What about if it is #1?
Can we just do rat MCAO first and test the behavioral tests used in SPAN1 to further verify these behavioral tests? Cylinder test, tape removal, and foot fault are supposed good behavioral tests for long-term stroke studies.
For embolic mouse MCAO, I found that Iowa's group only let the mice survive for 7 days and Augusta's group let the mice survive for 48 hours. Probably, we should use the rat model as well.
I agree with you and Anil…
I agree with you and Anil that we should do both rats and mice in the pilot to ensure we choose behavioral tests that are sensitive and reproducible across labs in both species. One finding that Cenk reported from his analysis was that waking mice up during occlusion actually led to smaller strokes (not larger as we expected from neuroprotection from anesthesia)- we think that is because the filament may have moved a little it as animals were moving around, but really don't know for sure.
We do have foot fault data from our grid walking setup from SPAN 1.0 that we should look at closely.
I'm very interested in the extended neurological scoring you developed, Y maze, and the phenotype box that Bingren showed.
Huaxin, In fact, I think…
Huaxin,
In fact, I think the problem was also #1, perhaps even more so than #2. The infarcts were too small in both groups.
After seeing the control…
After seeing the control cohort data and other ongoing projects in the lab where we used the SPAN protocol, I am strongly opposed to awake state during MCAO. The cause of smaller infarcts when you wake them up is likely premature reperfusion due to filament movement and leak as well as higher arterial pressures in animals awakening with sutured incisions etc. despite pain control.
Awake or sleep
Temperature is another factor affecting the stroke outcome. If the temperature is not controlled during awake MCAO, we have to make sure the heating will be switched off and on at the same pace in all mice.
We had one experiment in which we controlled the temperature during awake MCAO. A soft probe was implanted for temperature control and animals carried a swivel device so the temperature probe would not be pulled out. The infarcts were larger when the rats were kept awake during MCAO. We had to decrease the ischemic duration from 90 minutes to 75 minutes in that experiment.
We invariably saw smaller…
We invariably saw smaller infarcts in awake animals. Very recently, for a different project, we used the SPAN filament MCAO protocol but kept the animals awake and MCAO for 90 minutes. Infarcts were smaller than when we kept them anesthetized and only 60 min MCAO.
Possible reasons are filament instability causing premature reperfusion during animal movement and hypothermia (more relevant for mice) while awake because we cannot control the temperature, and they are not as physically active as before the stroke.
In rats, the situation may be different.
PERHAPS SOMETHING WE COULD TEST IN THE PILOT? Half of the rats and mice at each site could be awake and half anesthetized during MCAO.
blood pressure
We know that blood pressure is higher in the awake state. If the filament you used only induces a small infarct, another filament coated with a little long silicone should be considered in awake mice. We can test it in the pilot as well.
I agree on all the issues…
I agree on all the issues with small infarcts when awakening the mice. I think the main drawback to keeping animals asleep the whole time is the number of surgeries per day that can be done in the rats given the 2 hour occlusion time. Agree with Huaxin, about the potential filament size confounder. We should look at that SPAN 1.0 data carefully together as we make these decisions. We probably have the data to answer the question without including as another variable in the pilot
Here is some feedback and…
Here is some feedback and queries from MGH regarding the 48-point scoring system upon viewing the video:
1. Many of the scoring points are subjective, not easy to evaluate, difficult to differentiate between different grades (e.g., somnolent vs calm).
2. The test will be done in mice but videos are from rats. Will it be as easy to evaluate mice for the same scoring points?
3. The test is labor-intensive, takes too long.
4. Do we need baseline assessment or will we assume perfect performance at baseline? Do we need to wait a certain time between different sections of the test in the same animal?
We will be performing a mock exam in one mouse as requested and we will let you know how long it takes in our hands and if we have any additional feedback.
how to score them
NIH Stroke Scale-like Neurological Score System (0 = normal and 48 = severe)
How to score the animals using this scoring system? See below.
General Status (0-12)
The animal is placed in a new cage with bedding and watched for 5 minutes.
0 point: Brisk, can stand up by holding walls
1 point: Walks but can’t stand up by holding walls
2 points: Can walk, but trends to rest
3 points: Can’t walk, can look around
4 points: No movement
0 point: Posture shift to both sides
1 point: Head to one side, rarely head to the other side
2 points: Posture shifts to one side continuously
3 points: Posture shifts to one side, but hardly walks
4 points: does not walk
The animal is placed on the table surface and watched.
0 point: Walk freely, no abnormality
1 point: Supports left extremities by right
2 points: Clear limping or body swinging
3 points: Difficult walking
4 points: Does not walk
Motor Functions (0-22)
The animal is on the table. The tail is held up, and the front legs are allowed to touch the table surface.
0 point: Stretches both arms symmetrically
1 point: Asymmetry, difference less than 45 degrees
2 points: Asymmetry, difference more than 45 degrees
3 points: Asymmetry, can’t extend left arm
4 points: No movement in left arm
The animal is placed on the table and allowed to walk freely.
0 point: Turns to both sides equally
1 point: Tendency to turn to one side, can walk straight
2 points: Circles to one side, makes a large circle, can’t walk straight
3 points: Circles constantly to one side, make a small circle
4 points: Circles to one side without walking or no movement
The animal is on the table. The tail is held up, and only the front limbs are allowed to walk on the table surface.
0 point: Walks in both directions
1 point: Tendency to circle to one side, occasionally turns to the other side
2 points: Circles to one side
3 points: Circles to one side sluggishly
4 points: Does not advance, no movement
The animal is on the table. The hind limbs are left outside the table.
0 point: Immediately place on the table
1 point: Slowly place on the table
2 points: fail to place on the table
The animal is placed on the screen, which is then turned almost vertically.
0 point: Completes climbing, shifts to both sides
1 point: Climbs and shifts to one side
2 points: Holds on slope but does not climb
3 points: Slides down slope with some effort
4 points: Slides immediately, no effort to prevent fall
The animal is placed in the middle of the beam and allowed to walk.
0 point: Reaches up to the end wall
1 point: Can walk on the beam but does not reach up to the end wall
2 points: Can’t walk on the beam but stays more than 10 seconds
3 points: Stays on the beam less than 1o seconds
4 points: Fall immediately without any effort to prevent fall
Sensory functions (0-14)
The animal is placed on the table and allowed to move freely. Use the needle to touch when the animal is not walking.
0 point: Brisk on both sides
1 point: Slow left and brisk right
2 points: Absent left and some responses right
0 point: Brisk on both sides
1 point: Slow left and brisk right
2 points: Absent left and some responses right
Animal is placed inside the cage. Use the needle to touch the truck, vibrissae, and face.
0 point: Brisk bilaterally
1 point: Slow left and brisk right
2 points: Absent left and brisk right
3 points: Absent left and slow right
4 points: Absent bilaterally
0 point: Brisk bilaterally
1 point: Slow left and brisk right
2 points: Absent left and brisk right
3 points: Absent left and slow right
4 points: Absent bilaterally
0 point: Brisk on both sides
1 point: Slow left and brisk right
2 points: Absent left and some responses right
pilot study
I vote to do the rat model in the pilot study. Rats do not have the PcomA issue so the coated silicone can be a little longer so we can do the awake MCAO if we want. In the mouse model, we use the filament coated only with short silicone (do not like block the blood flow to PCA) so the blood flow to MCAO will not be well blocked if the filament is shifted during ischemia in the awake state. More importantly, the mortality is low in the rat model.
48-point scoring system
After watching the behavioral test video. Here are some comments from Augusta sites:
1. Scoring systems were exhaustive and time-consuming. Their activity may change early morning vs late noon. Also, the behavioral activity grades here are not mentioned whether it is performed in regular or reverse light cycles of rodents. So, counting all these 48-point grades look time-consuming and not easy to evaluate. If we plan to do more behavioral testing mice will get exhausted and stressed. In most of the tests, we need to handle mice or rats which is not good if we need many tests in a day. If we want to use this 48 points scoring we need to minimize the number of testing. From the suggested testing we can do fewer tests such as:
General status: Spontaneous activity and body symmetry
Motor function: Front limb symmetry and Circling
Sensory function: Vibrissae touch
48-point scoring system
The spontaneous activity may change early morning or late afternoon. However, when you put them in a new clean cage, they always walk around. For beginners, it may take time to get it done. In fact, just a few steps: watch or test them inside a new cage, watch or test them on the table, beam walking, and screen climb. These activities are essential and not affected by the light cycle.
90 min mouse MCAO
Have you done any long-term survival studies in mice using 90 min filament MCAO model? If yes, please share some information about the filament size, body temperature, and mortality.
Thanks,
Huaxin
90min MCAO in mice
Hi!
I have not done any long-term survival studies in mice using 90 min filament MCAO model.
Thank,
Pradip
The mortality rate could be…
The mortality rate could be high in long-term survival studies in mice if using 90 min filament MCAO model. However, we have not done this yet in mice.
Long term survival
I have done a study comparing 30, 60, 90, 120 and 3h, 6h and 12h occlusion times in a rat MCAO model. We use heat blunted suture and we occlude the CCA but we wake them up during occlusion. we had one death during stroke, one in the 30min group, and one in the 3h group, but no deaths in the other groups. Rats are very hardy.
Long term survival
Yes, it can be in rats. We have done 90 min occlusion in rat MCAO models in another study. But not in the mouse.
All of us have no problem…
All of us have no problem with 90 min MCAO in rats. However, it is not easy to do it in mice. We use a transcranial MCAO mouse model for long-term survival experiments. The model can be used for several hours of ischemia and the mortality is almost zero. I do not know if you like to use it here.
Here is the feedback from…
Here is the feedback from MGH regarding the 48-point test.
Sanem and Taka did one mouse (C57 day 2 after 1h filament MCAO). The test took 60min to run it, also 20-30 min habituation plus recording every video may take a total of 90 min.
Concerns & Thoughts:
MGH feedback from clot…
MGH feedback from clot preparation trial by Taka:
We did not have fibrinogen on time so we did without.
Blood sample was collected from both a vein and an artery. Venous blood clot was stable, arterial tended to fragment easy. Yellow tape is under the arterial clot. Washed 15 times. It seems easy to make the clot but whether we can successfully suck into the catheter and inject into ICA remains to be seen.




I had images but the web…
I had images but the web platform rejected them.
48 point score
From my team:
Ligia and I just completed testing of the Duke behavioral battery on a healthy WT young male mouse. Our observations and recommendations are listed below. Overall, the test seems really promising and a much more accurate reflection of clinical outcomes, but the challenges of translating everything from rats to mice, as well as the time/effort that would be required both to perform and to rate the videos, is pretty high.
- Only tried on a non-stroked animal, so many of the tests are much more difficult to do than they would be with an animal who has stroke-related deficits. We should probably try it again with a stroked animal and compare the two.
- Testing clearly requires two camera angles — one from the top, and one from the side. Each test is better viewed from one or the other. Would maybe make sense to film all the 'from the top' tests at once and then all the 'from the side' tests at once, if moving around the order of the tests is allowed?
- Took us 25 minutes from start to finish for one animal, while consulting video for how to do tests and not doing the complex motor tests. Would guess that 15 min per animal is reasonable once someone is trained, especially if they don't have to score as they go.
- A lot of ambiguity on how long each test should take, how many trials are needed, etc. A detailed SOP would be incredibly useful.
- Mouse was clearly very stressed about midway through the tests, which is a concern. The video recommends waiting a few minutes for the mouse to calm down, but this would need to be balanced against increasing the length of evaluation.
- Many of the tests are done on an elevated countertop. Would need to design a 3-sided box or some other structure so that the mice don't move out of camera range.
OBSERVATIONS ON SPECIFIC TESTS ARE BELOW
General Status:
- Seems super easy to do General Activity and Body Symmetry tests — can film from top. They suggest recording for 5 minutes, but we both feel you could get the same info with a 1-2 min video.
- Gait Walk test needs lateral recording and some sort of box so animals don't escape.
Motor Tests:
- Front Limb Symmetry and Front Limb Circling could possibly be combined into one recording. More difficult to do with mice because holding them at the exact right height is challenging. Needs lateral recording.
- Circling test relatively straightforward, would be recorded from the top.
- Hind Limb Placement test seems really difficult to do with a mouse - feels like they're going to fall when you put them on, and is hard to assess. Also would require a complicated camera setup.
Complex Motor Tests:
- Tried a modified version of the Screen Climb test using one of the grid walk grids. Seemed to work pretty well. Couldn't try out the Wooden Beam test.
- Would need standardized, non-wood equipment for both the Screen Climb test and Wooden Beam test if we're going to use it in Gold Standard.
Sensory Tests:
- Very, very difficult to assess with a non-stroked animal. The mouse constantly runs away, making evaluation or repeated trials quite challenging. Difficult to isolate the effect of sensory stimulation from general skittishness.
- A lot harder to separate facial from whisker stimulation in mice than in rats, especially if the animal is healthy.
- We used a Q-tip, rather than the blunt 30G needle they recommend. Seemed to elicit a response, but the relative sharpness of the needle may be a more salient stimulus.
- Forelimb and hindlimb tests are probably best to film laterally, while the face, vibrissae, and back tests can all be filmed from the top.
Ligia and I just completed testing of the Duke behavioral battery on a healthy WT young male mouse. Our observations and recommendations are listed below, and you can find videos of some of the tests here. Overall, the test seems really promising and a much more accurate reflection of clinical outcomes, but the challenges of translating everything from rats to mice, as well as the time/effort that would be required both to perform and to rate the videos, is pretty high.
- Only tried on a non-stroked animal, so many of the tests are much more difficult to do than they would be with an animal who has stroke-related deficits. We should probably try it again with a stroked animal and compare the two.
- Testing clearly requires two camera angles — one from the top, and one from the side. Each test is better viewed from one or the other. Would maybe make sense to film all the 'from the top' tests at once and then all the 'from the side' tests at once, if moving around the order of the tests is allowed?
- Took us 25 minutes from start to finish for one animal, while consulting video for how to do tests and not doing the complex motor tests. Would guess that 15 min per animal is reasonable once someone is trained, especially if they don't have to score as they go.
- A lot of ambiguity on how long each test should take, how many trials are needed, etc. A detailed SOP would be incredibly useful.
- Mouse was clearly very stressed about midway through the tests, which is a concern. The video recommends waiting a few minutes for the mouse to calm down, but this would need to be balanced against increasing the length of evaluation.
- Many of the tests are done on an elevated countertop. Would need to design a 3-sided box or some other structure so that the mice don't move out of camera range.
OBSERVATIONS ON SPECIFIC TESTS ARE BELOW
General Status:
- Seems super easy to do General Activity and Body Symmetry tests — can film from top. They suggest recording for 5 minutes, but we both feel you could get the same info with a 1-2 min video.
- Gait Walk test needs lateral recording and some sort of box so animals don't escape.
Motor Tests:
- Front Limb Symmetry and Front Limb Circling could possibly be combined into one recording. More difficult to do with mice because holding them at the exact right height is challenging. Needs lateral recording.
- Circling test relatively straightforward, would be recorded from the top.
- Hind Limb Placement test seems really difficult to do with a mouse - feels like they're going to fall when you put them on, and is hard to assess. Also would require a complicated camera setup.
Complex Motor Tests:
- Tried a modified version of the Screen Climb test using one of the grid walk grids. Seemed to work pretty well. Couldn't try out the Wooden Beam test.
- Would need standardized, non-wood equipment for both the Screen Climb test and Wooden Beam test if we're going to use it in Gold Standard.
Sensory Tests:
- Very, very difficult to assess with a non-stroked animal. The mouse constantly runs away, making evaluation or repeated trials quite challenging. Difficult to isolate the effect of sensory stimulation from general skittishness.
- A lot harder to separate facial from whisker stimulation in mice than in rats, especially if the animal is healthy.
- We used a Q-tip, rather than the blunt 30G needle they recommend. Seemed to elicit a response, but the relative sharpness of the needle may be a more salient stimulus.
- Forelimb and hindlimb tests are probably best to film laterally, while the face, vibrissae, and back tests can all be filmed from the top.
Further feedback from Taka…
Further feedback from Taka on the 48-point model:
The animal we studied (as above) showed mild deficits (many sub-score 1-2). TTC showed good stroke volume. If stroke sizes are smaller, it maybe difficult to judge the score.
Yes. Most stroke animals…
Yes. Most stroke animals have a sub-score of 1-2. In our 90 min MCAO rat experiment, the mean neurological scores at 7 days post-stroke were 16 in the vehicle group, and 10 in the treatment group. At 28 days post-stroke, the scores were 12 and 7 in the vehicle and treatment groups, respectively.
I shared link to a google…
I shared link to a google drive folder with videos from our piloting of the Duke protocol with Jessica and Karisma, hopefully they can share with the emails the PIs are able to access google drive with (I think some were using different emails due to university restrictions and I don't know who or what they are).
the pilot study
Our lab can do the mouse MCAO model using both awake or anesthetized mice. However, the mouse MCAO model is more reproducible in anesthetized mice than in awake mice. If a drug testing in an individual lab, I would highly recommend using anesthetized mice and monitoring/measuring the MCA blood flow during the entire peri-MCAO periods. However, the multiple site testing might overcome the reproducibility issue as more mice will be used for the study.
New link to Yale video of 48 pt scoring
https://drive.google.com/drive/folders/1yBfAP-drVeJ7IKCLtzDQ-PqwMo_Gpljn?usp=share_link
suture info from UCSD
Hi Pat,
Thank you for hosting the stimulating discussion of the span SOPs. Below is our consideration of the filament occluder size: The monofilament suture occluder size is important in producing a reproducible MCAO and reperfusion. Minor alterations in the suture silicone coating diameter can greatly affect stroke brain injury volume and reproducibility in this model. In our studies, we use a body weight-to-diameter scale method; the lower the body weight in animal, the smaller of a silicone coating tip diameter is used. For the mouse model, our mouse surgeons prefer the Syneture™ Monosof™ SN5696G 6-0 suture because of the suture quality and consistency. The suture tip is coated with silicone to different sizes in diameter. For example, a 0.22-0.23 mm diameter range is used for 3 months old B6 male mice (23-28 g). A 0.19-0.21 mm diameter range is used mostly for 3 months old B6 female mice (17-22 g). When a larger size of suture tip is used for a smaller animal, the suture tip may not reach to the MCA base or punch through the vessel wall before reaching the MCA origin, resulting in either incomplete occlusion of MCA or subarachnoid hemorrhages.
In our experience, smaller mouse body weight variations in a range of 23-28g, the 0.21-0.23 mm tip width may not make any difference in the infarct volume. However, when mice are significantly smaller such as female mice, a small tip width may offer more reproducible result.
Have a greta weekend!
Bingren
the pilot study
It is very useful to use a needle laser doppler probe to monitor the MCA blood flow while inserting the monofilament occluder to the MCA base as the MCA blood flow sudden drop can clearly indicate that the occluder reaches the MCA base. When a filament occluder tip is >0.22 mm for smaller mice such as female mice (body weight about 19-20 g), the occluder may not be able to reach to the MCA base. This can be seen by monitoring the MCA blood flow as the MCA blood flow will not drop even when the inserting resistance is felt by the surgeon. In this situation, inserting with more force will likely punch through the vessel wall before reaching the MCA origin, resulting in either incomplete occlusion of MCA or subarachnoid hemorrhages.
Clot model
The mice are on Apoe-/- background (hyperlipidemic).
Clot model
The mice are on Apoe-/- background (hyperlipidemic).
mild MCAO
Two mice had 20 min MCAO and only one had a small infarct. Another two mice had 30 min MCAO and both has a small infarct. It seems like we should use 30 min MCAO in the pilot study.
mild MCAO
Nicely done, Huaxin. May I ask if you anesthetized the mice and continuously monitored the MCA blood flow during the peri-MCAO periods (pre-, during, and post-MCAO)?
mild MCAO
Yes. Mice were anesthetized with 1.5% isoflurane in 30% O2 and 70% N2O. We used the 602223 filaments and monitored blood flow during ischemia. The blood flow was reduced to 40% of the baseline when CCA was open.
pilot study
40% of MCA blood flow during MCAO related to the pre-ischemic baseline? Is the 40% too high? We temporally clamp/close CCA during MCAO and the MCA blood flow during MCAO is below 10% of the pre-ischemic baseline.
The blood flow was reduced…
The blood flow was reduced to below 20% when the CCA was closed.
Pilot Study
Augusta site did 20 min and 60 occlusions. Twenty min has no infarction after TTC, whereas 60 min has good infarction.
Date of Surgery: 04/18/2023
Mice male: 11-12 weeks
Body weight BL: 28-30gm
Suture used: 0.23
Sleep surgery
CAA: Closed
CBF after 20 min & 60 min
TTC for 20 vs 60 min occulusion
MGH Behavioral Testing Comments (Yale)
Videos recorded far away from the subject & are not easy to score. Mouse 1 circling video is too short, and the mouse disappears from the video.
General status videos:
Difficult to differentiate between scores, 1/2 points + 3/4 points, which looked very similar to us.
We suggest a new scoring scale (new grades in blue.) 5 minutes is too long, 1–3-minute duration should be enough.
0 points: Normal (0)
1 Point: Calm (1)
2 Points: Somnolent (1)
3 Points: Stuporous (2)
4 Points: No spontaneous movement (2)
Symmetry videos:
Body symmetry videos are similar to circling/gait videos. Only forelimb symmetry can be checked.
Grading (Gait) videos: Videos recorded at too great of a distance. Not easy to score and observe the animal.
Circling bench top videos: Mouse 1 video was short, could not even evaluate the circling. During the gait video, circling can be evaluated with no need to separate the tests.
Circling hold tail: This is similar to the forelimb symmetry test. If the mouse is circling asymmetry will occur.
Hind limb placement: Mouse is much lighter than a rat, will be difficult to conduct this testing.
Screen climbing: MGH team approves of this testing strategy. We’ll need specifications of the testing apparatus (gap size, angle, height) to prepare MGH spaces for this test. The 0-4 score looks good.
Beam walk: We will require beam specifications. Beam walk test must be performed 3 times – how will we conduct scoring if the animal does not fall during first trial? What if animal falls on second or third attempt?
Forelimb touch and Hindlimb touch: This sensory test is not easy to assess because, unlike rats, mice have small limbs. This complicates graders’ ability to score videos. Videos are recorded from too great a distance and are not easy to evaluate. Sensory testing tools must be standardized- MGH staff noted differences between the equipment used by Yale and Duke.
Trunk, vibrissae, and face touch: Again, videos are taken from too great a distance. Not easy to evaluate. We think this test is helpful to use. Video recording should be standardized to maximize evaluation success.
We propose the Garcia 18-point scoring test, published in 1995, as an alternative to the 48-point testing proposed by SPAN CC. MGH believes that the Garcia 18-point test will cover all aspects of the 48-point scoring test.
(https://www.ahajournals.org/doi/10.1161/01.STR.26.4.627,https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00316/full)
MGH Team
Garcia's score and 48-point score
The 48-point score was developed from a combination of Garcia's and other scores.
Garcia's and other scores
We compared different scores (A=48-point score, B=Garcia Score, and C=Bederson score).
circling
The circling behavior is included in the 48-point score system. We took one old experiment, mixed all treatment animals, and analyzed the circling behavior separately. The circling behavior was associated with the infarct size (p=0.03). However, the neurological score represented the infarct size better.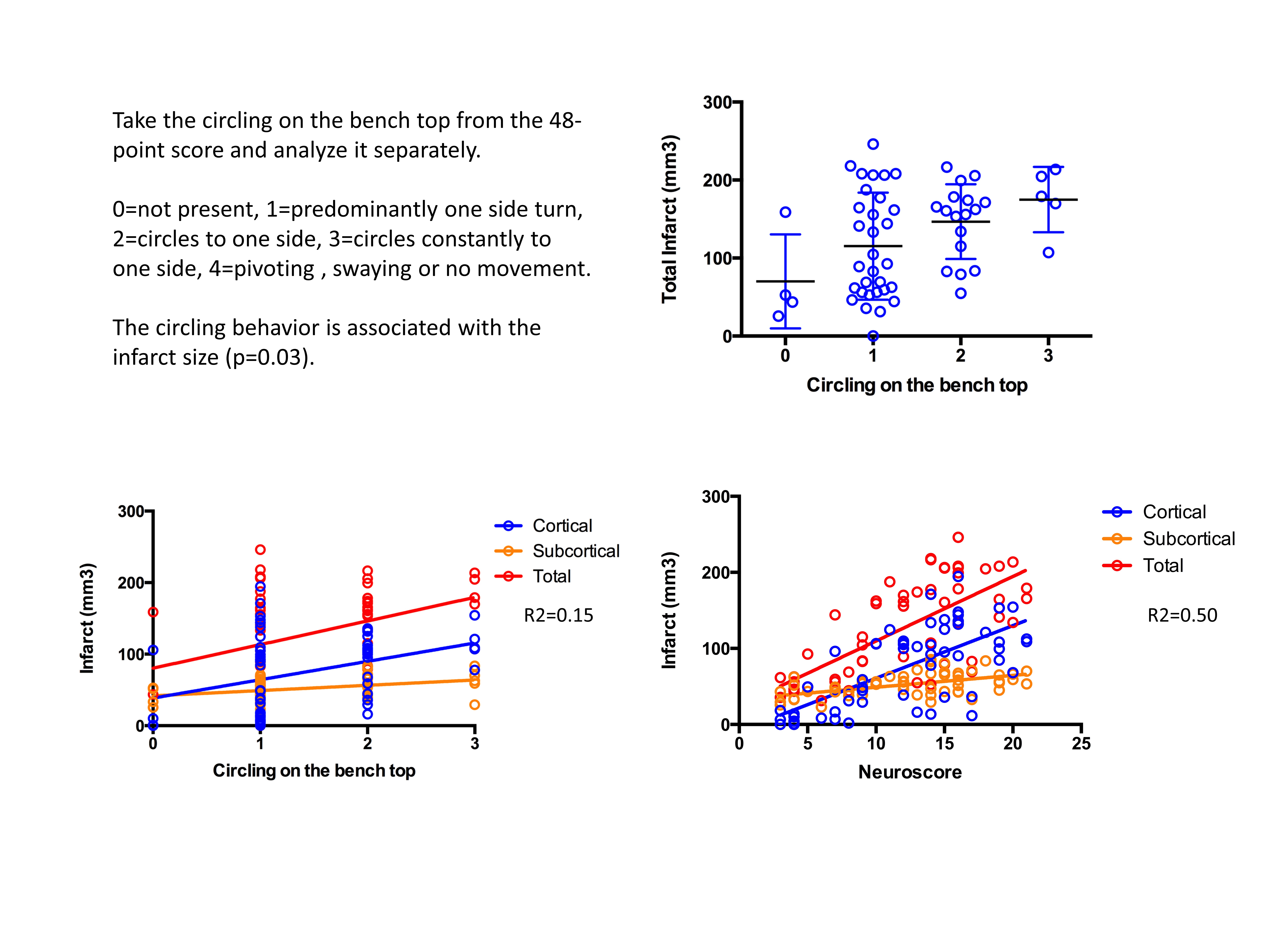
AUGUSTA site Pilot study: 30 min vs 40 min
AUGUSTA site Pilot study: 30 vs 40 min_infaraction volume_CBF
AUGUSTA site Pilot study: 30 vs 40 min_Bw, corner test_hanging w
corner test
There was a significant infarct volume difference between these two groups. However, the corner test result was not significant. Is there a clear answer to this? The corner test will serve as the primary outcome of our pilot study, as we discussed in Friday's meeting.